Navigating Clinical Trials
Clinical trials can be a complex process that involves many steps to move from an idea to implementation and dissemination of results. OCRA can assist researchers with determining when a study is a clinical trial, developing clinical trial designs for implementation, and navigating various regulatory requirements governing these studies.
The NIH revised its definition of a clinical trial in 2014 as part of a multi-year, comprehensive effort to bring more public transparency and accountability to biomedical research. Consequently, many human subjects studies that previously would not have been considered a clinical trial now fall under the new definition.
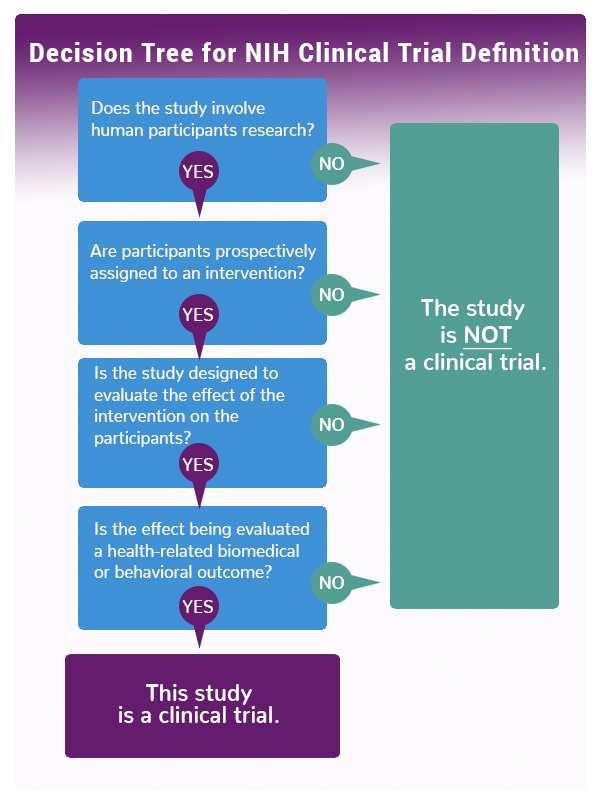
NIH defines a clinical trial as “a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.” As the decision tree graphic indicates, if you answer “yes” to these four questions, the NIH considers your study a clinical trial.
To assist researchers, the NIH has identified various types of clinical trials:
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Screening trials test new ways for detecting diseases or health conditions.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with conditions or illnesses.
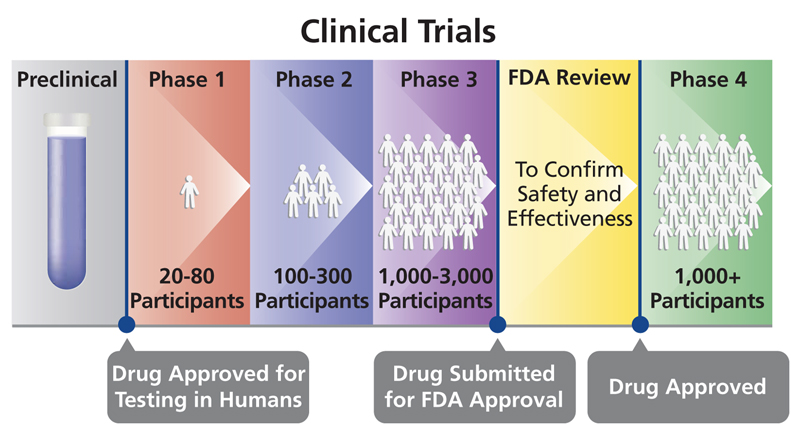
There also are defined phases of clinical trials that focus on answering different questions about the treatment, drug, device or behavioral intervention being studied. It can take years for a study to go through the phases and gain approval by the U.S. Food and Drug Administration (FDA):
- Phase I trials: Researchers test a drug, device, treatment or intervention in a group of people (under 100 participants) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
- Phase II trials: The new drug, device, treatment or intervention is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
- Phase III trials: The new drug, device, treatment or intervention is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
- Phase IV trials: After the discovery has successfully navigated the previous phases, is approved by the Food and Drug Administration and made available to the public, researchers track its safety in the general population, seeking more information about benefits and optimal use.